dermaPACE
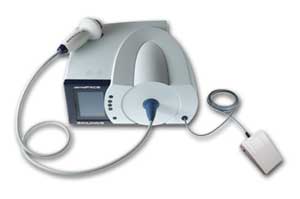
The dermaPACE device system is cleared by the U.S. Food and Drug Administration (FDA) for the treatment of diabetic foot ulcers and is available for placement in the US. The dermaPACE is CE Marked for select international markets for the treatment of chronic and acute conditions of the skin and subcutaneous tissue.
